Cell-Signalling and Bio-Imaging Unit (CSBU)

The Department of Biological and Biomedical Sciences houses a variety of state of the art microscopy systems housed in well-equipped and recently refurbished Charles Oakley Laboratories.
 Systems available include:
Systems available include:
Confocal and Live cell imaging:
Zeiss inverted microscope with: LSM800 AIRYSCAN confocal system (Zen imaging software)
Olympus inverted microscope with: Crest X-light Spinning Disk Confocal, Ilas2 TIRF illuminator and metamorph for real-time super resolution microscopy
Calcium imaging and Microinjection:
Nikon Eclipse TE2000U inverted microscope with:
Photometrics coolsnap HQ camera
Cairn Research OptoLED rapid calcium imaging work station
Microinjector – Eppendorf Femtoject InjectMan N12
Phase contrast and Histological Analysis
Zeiss Axiovert 200 linked up to Axiocam 105 color camera (Zen Imaging software)
EVOS AMG fluorescent microscope
Contact
For further information please contact:
Dr Patricia Martin
Email: patricia.martin@gcu.ac.uk
Testimonials
Confocal Microscopy
Assistant Professor, Fralin Biomedical Research Institute at Virginia Tech (USA)
Research link for Scott R. Johnstone
I started at GCU with measures of intracellular calcium with Dr Douglas Bovell during an undergraduate summer internship. This is a skill I still use today, and was the subject of one of our more recent papers (Yang, J. Immunol 2020). Starting my PhD with Dr Martin in 2004, I was one of the original users of the Bioimaging facility at GCU. I was trained in the use of widefield and confocal imaging, using the facilities in the bioimaging core to investigate the nature of proteins involved in cellular communication and cell proliferation.
The bioimaging core at GCU, and the training it provided me, gave me an excellent understanding of microscopy which I have relied upon through my research career. Imaging plays an integral role in my research and my lab is highly dependent on our own confocal intravital microscope that we use for investigations of vascular disease. We rely upon multiple imaging approaches from serial block face electron microscopy, confocal microscopy, intravital microscopy and stochastic optical resonance microscopy (STORM) to visualize the functions of proteins in cells and their impact on disease.
The training provided to me at GCU with the experience using the bioimaging core has been essential to my ongoing success throughout the years and has led to over 40 publications, many of which were reliant on effective imaging techniques.
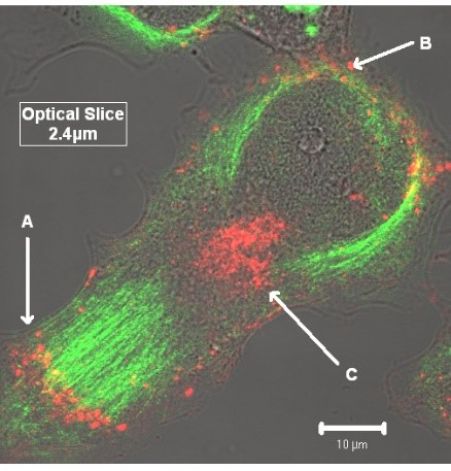
Favourite image: My first is an image from the Bioimaging core taken in around 2007 – it shows the disruption of microtubules by taxol and the differential localization of proteins (Red, Cx43) on the microtubules (green) – This was an important image in my career as it demonstrates very clearly the effects of the drug taxol which is used in drug eluting vascular stents. Taxol stops cellular processing of proteins including the delivery of connexins to the cell membrane. In our research we have shown that Cx43 trafficking is an essential component of vascular disease and that current drugs used, such as taxol , may cause more damage resulting in long term failures.
Current postdoc at Heriot-Watt University, Edinburgh (Edinburgh Super Resolution Imaging Consortium & Institute of Biological Chemistry, Biophysics and Bioengineering)
BSc(Hons) Cell & Molecular Biology, GCU, 2012-2016
The Bioimaging Unit at GCU gave me my first taste of advanced optical microscopy methods and how they were applied to the study and treatment of disease. I used the Unit's Zeiss LSM800 confocal laser scanning microscope to visualise the localisation pattern of connexin proteins and mutants in various cell lines during my Honours research project. The expert training and practical experience I received in the Bioimaging Unit sparked my interest in microscopy development and applications to biomedical research - which led me to transition into an interdisciplinary PhD building microscopes and applying them to numerous biological questions.
Since that catching that first spark for imaging at GCU, I've gone onto develop and apply many advanced imaging methods spanning interferometric microscopy, high-resolution mesoscopy, super-resolution and single-molecule localisation microscopy, and developing open-source microscopy instrumentation. I've also been more and more involved in the field via the Royal Microscopical Society (RMS), which I found out about during my time at GCU, and I am the current Chair of the Early Career Committee, member of the Life Sciences Section Committee, and of the RMS Council. My career path would not have been on this trajectory without the imaging experience I gained during my final year at GCU.
PhD student - Glasgow Caledonian University 2018-2021
“Confocal microscopy illuminated my PhD journey, giving me infinite and multiple starry skies to admire.”
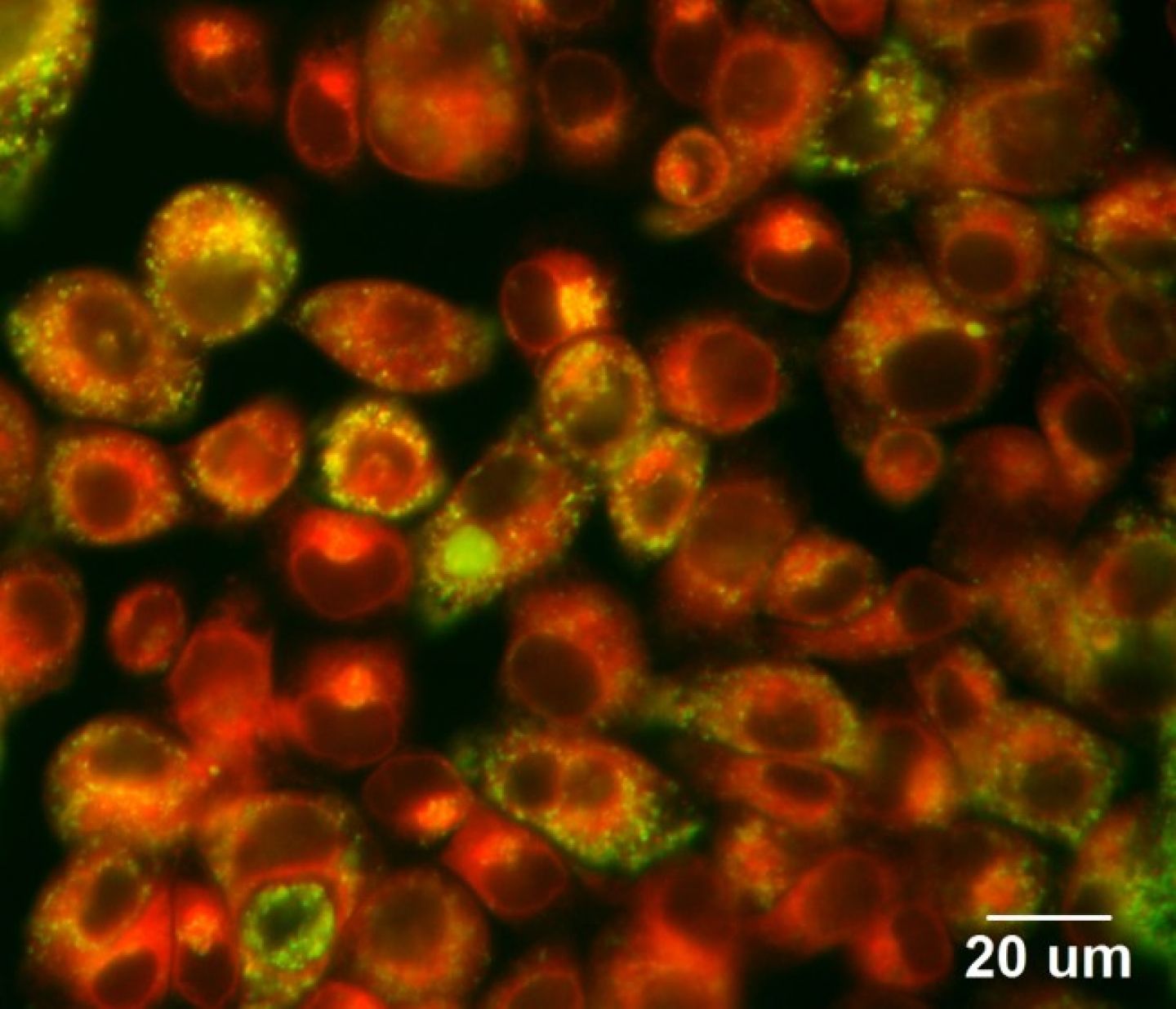
Favourite image: apocrine sweat gland cell line stained with b-tubulin and Cx43.
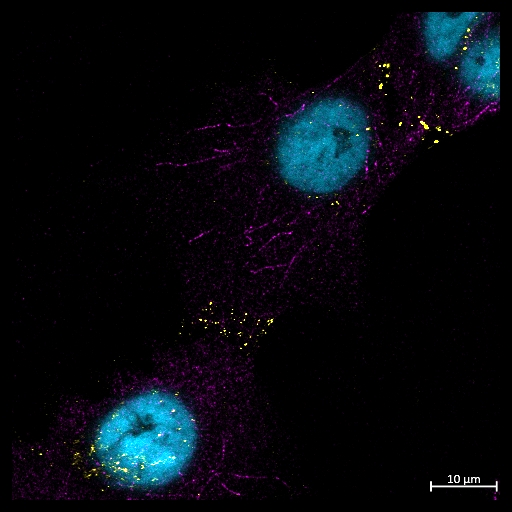
Year 1, PhD student – Glasgow Caledonian University 2022
An amazing facility that is crucial to conducting my research. I always look forward to experiencing the beauty of nature through imaging my cardiomyocytes with the confocal microscope.
Favourite image: Confocal Image of Rabbit Right Ventricular myocyte, stained with Ryanodine receptor and Caveolin-3 antibodies.
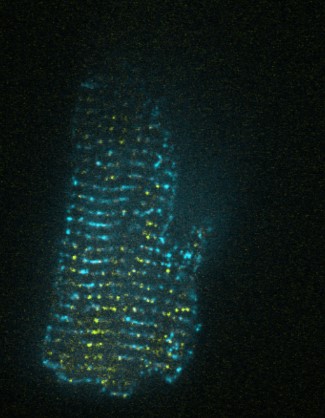
Cell biology lecturer, Glasgow Caledonian University
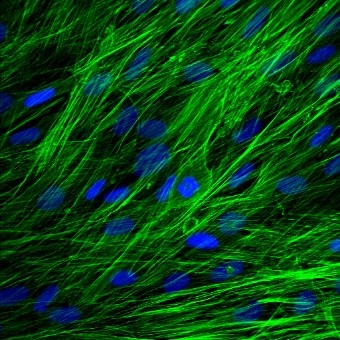
I am a user of the Bioimaging suite confocal microscope, which I have used for fixed and live imaging of cells (live staining, cell migration). This has been essential to my work and allowed me to demonstrate high resolution localisation of gap junctions and other proteins in 2D and 3D cell models. I have also used the calcium imager and EVOS fluorescent microscope widely.
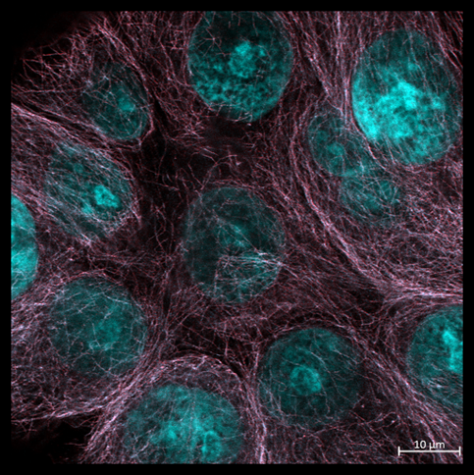
Favourite image: Phalloidin staining of actin in human skin fibroblasts.
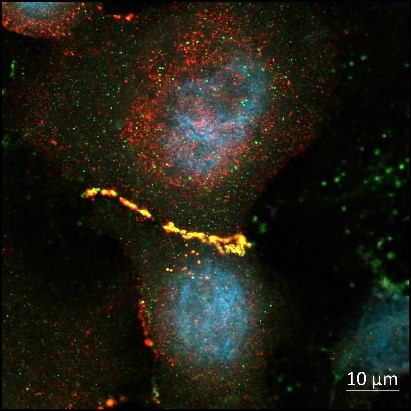
British Skin Foundation funded PhD student – University of Glasgow
As a joint PhD student between Glasgow Caledonian University and Glasgow University, I have gained new skills that have been crucial to my research using the microscope suites at Glasgow Caledonian University. Dr Boatemaa Ofori-Frimpong has provided training for this.
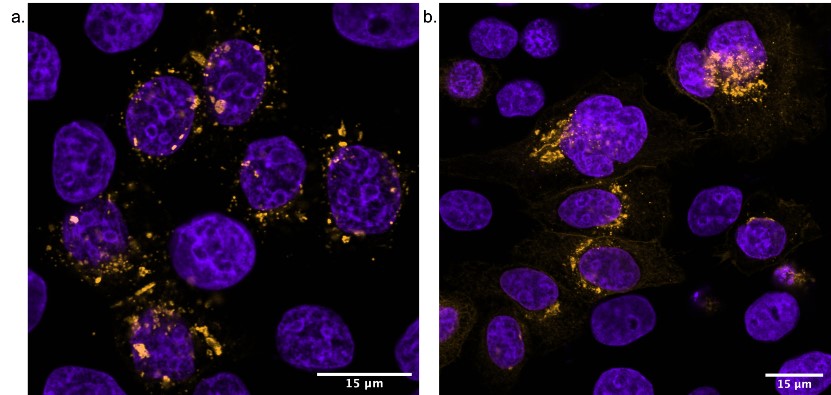
Favourite image: human keratinocytes from a diabetic patient co-stained with Cx43 and Zo-1 indicating co-localisation at points of cell to cell contact.
Flow Cytometry
Neret Pujol-Navarro, PhD student at the University of Strathclyde
‘As a PhD student from the University of Strathclyde, I have gained new skills using the flow cytometry facilities at Glasgow Caledonian University. Dr Mark Williams (GCU), has been key to providing the necessary training as he is an expert in this technique.’
Katerina Miari, PhD student at Glasgow Caledonian University
‘I use the flow cytometer on a weekly basis to obtain high-quality data for my thesis and future publications. During my PhD I have successfully optimised various flow cytometry techniques with the help of my supervisor Dr Mark Williams who runs the flow cytometry facility (e.g., apoptosis assays, cell cycle analysis, purity assessment of cell populations'
Saeed Alqahtani, PhD student at the Dental School, University of Glasgow
“I am a PhD student from the University of Glasgow who chose to run his samples at Glasgow Caledonian University. The flow cytometry facilities lead by Dr Mark Williams has provided very valuable input in my project. Dr Mark and his PhD student Katerina had provided much needed insight in flow cytometry experiments where other facilities failed.”